the core of our corporate values
Zylox Medical Receives CE Mark for ZENFlex Peripheral Stent System
Zylox Medical today announced that ZENFlex™ Peripheral Stent System has received CE Mark and is commencing commercialization immediately in the European Union and other countries where CE Mark is recognized.
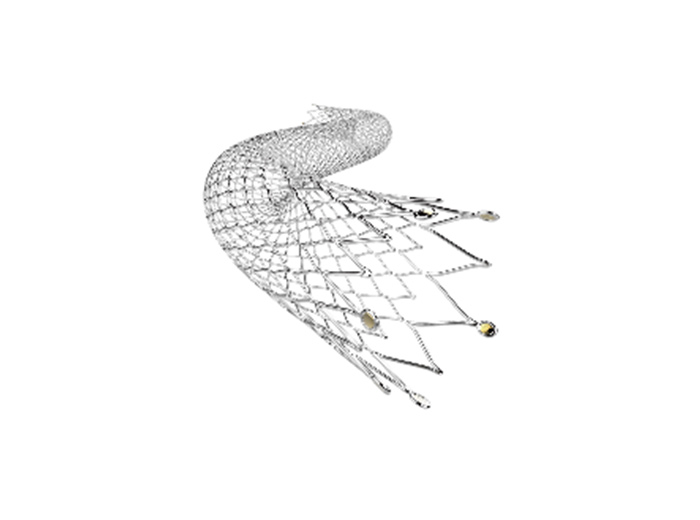
The ZENFlex™ Stent System is designed to restore blood flow in the peripheral arteries above the knee – specifically the superficial femoral artery and proximal popliteal artery .
The product features MicroMesh design and unique spiral design that balances stent flexibility, vascular compliance and radial force. The technologies provide improved stent performances through challenging lesions.
ZENFlex stent is the neuro medical device companies’ second product which obtained CE mark. In 2015, the CE mark first approved Zylox ZENFlow PTA Balloon for treatment of iliac, femoral, ilio-femoral, popliteal, infra popliteal, and renal arteries.
The CE approval of two products provides the precondition for Zylox Medical to penetrate the global market of peripheral intervention devices.
For more information, please visit http://www.zyloxmedical.com/