the core of our corporate values


The ZENFLEX® Peripheral Stent System is designed to deliver a self-expanding stent to the iliac artery, superficial femoral arteries and/or proximal popliteal arteries via a 6F delivery system.
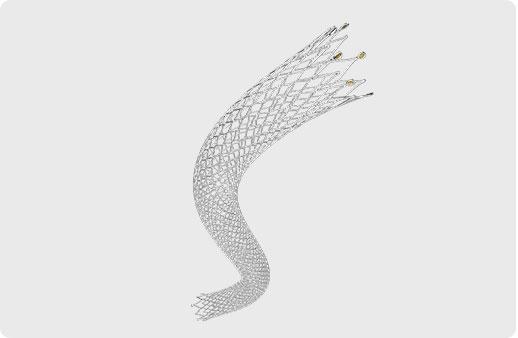

Unique Spiral Design
Excellent flexibility
Superior vascular compliance
Excellent Vascular Compliance
Every 2mm length contains 7 connecting bridges
MicroMesh Design
High radial strength and good fracture resistance
Maximizes vessel patency by decreasing tissue prolapse
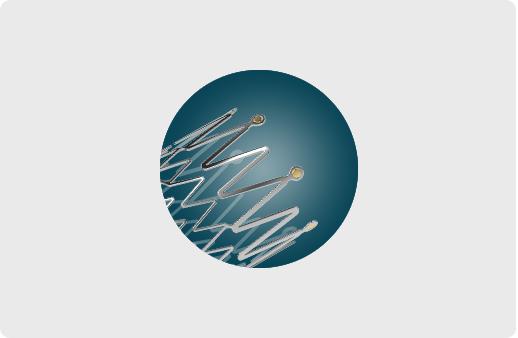
Gold/Tantalum Marker
Each end of the stent embedded 6 micro markers
Accurately mark stent ends out and enhances visibility
Controlled Release System
One-handed stent deployment
The initial precise positioning followed by well matching quick release
Ergonomic handle with anti-skid tuning dial combines comfortable hand feel and placement accuracy
Compatible with 0.035"guidewire


| Usable Length (800mm) | Usable Length (1250mm) | Stent Length (mm) | Stent Diameter (mm) | Usable Length (mm) | Sheath |
| ZX04020080B | ZX04020125B | 20 | 4 | 800/1250 | 6F |
| ZX04030080B | ZX04030125B | 30 | 4 | 800/1250 | 6F |
| ZX04040080B | ZX04040125B | 40 | 4 | 800/1250 | 6F |
| ZX04060080B | ZX04060125B | 60 | 4 | 800/1250 | 6F |
| ZX04080080B | ZX04080125B | 80 | 4 | 800/1250 | 6F |
| ZX04100080B | ZX04100125B | 100 | 4 | 800/1250 | 6F |
| ZX05020080B | ZX05020125B | 20 | 5 | 800/1250 | 6F |
| ZX05030080B | ZX05030125B | 30 | 5 | 800/1250 | 6F |
| ZX05040080B | ZX05040125B | 40 | 5 | 800/1250 | 6F |
| ZX05060080B | ZX05060125B | 60 | 5 | 800/1250 | 6F |
| ZX05080080B | ZX05080125B | 80 | 5 | 800/1250 | 6F |
| ZX05100080B | ZX05100125B | 100 | 5 | 800/1250 | 6F |
| ZX05120080B | ZX05120125B | 120 | 5 | 800/1250 | 6F |
| ZX05150080B | ZX05150125B | 150 | 5 | 800/1250 | 6F |
| ZX06020080B | ZX06020125B | 20 | 6 | 800/1250 | 6F |
| ZX06030080B | ZX06030125B | 30 | 6 | 800/1250 | 6F |
| ZX06040080B | ZX06040125B | 40 | 6 | 800/1250 | 6F |
| ZX06060080B | ZX06060125B | 60 | 6 | 800/1250 | 6F |
| ZX06080080B | ZX06080125B | 80 | 6 | 800/1250 | 6F |
| ZX06100080B | ZX06100125B | 100 | 6 | 800/1250 | 6F |
| ZX06120080B | ZX06120125B | 120 | 6 | 800/1250 | 6F |
| ZX06150080B | ZX06150125B | 150 | 6 | 800/1250 | 6F |
| ZX07020080B | ZX07020125B | 20 | 7 | 800/1250 | 6F |
| ZX07030080B | ZX07030125B | 30 | 7 | 800/1250 | 6F |
| ZX07040080B | ZX07040125B | 40 | 7 | 800/1250 | 6F |
| ZX07060080B | ZX07060125B | 60 | 7 | 800/1250 | 6F |
| ZX07080080B | ZX07080125B | 80 | 7 | 800/1250 | 6F |
| ZX07100080B | ZX07100125B | 100 | 7 | 800/1250 | 6F |
| ZX07120080B | ZX07120125B | 120 | 7 | 800/1250 | 6F |
| ZX07150080B | ZX07150125B | 150 | 7 | 800/1250 | 6F |
| ZX08020080B | ZX08020125B | 20 | 8 | 800/1250 | 6F |
| ZX08030080B | ZX08030125B | 30 | 8 | 800/1250 | 6F |
| ZX08040080B | ZX08040125B | 40 | 8 | 800/1250 | 6F |
| ZX08060080B | ZX08060125B | 60 | 8 | 800/1250 | 6F |
| ZX08080080B | ZX08080125B | 80 | 8 | 800/1250 | 6F |
| ZX08100080B | ZX08100125B | 100 | 8 | 800/1250 | 6F |
| ZX08120080B | ZX08120125B | 120 | 8 | 800/1250 | 6F |
| ZX08150080B | ZX08150125B | 150 | 8 | 800/1250 | 6F |
| ZX09020080B | ZX09020125B | 20 | 9 | 800/1250 | 6F |
| ZX09030080B | ZX09030125B | 30 | 9 | 800/1250 | 6F |
| ZX09040080B | ZX09040125B | 40 | 9 | 800/1250 | 6F |
| ZX09060080B | ZX09060125B | 60 | 9 | 800/1250 | 6F |
| ZX09080080B | ZX09080125B | 80 | 9 | 800/1250 | 6F |
| ZX09100080B | ZX09100125B | 100 | 9 | 800/1250 | 6F |
| ZX09120080B | ZX09120125B | 120 | 9 | 800/1250 | 6F |
| ZX09150080B | ZX09150125B | 150 | 9 | 800/1250 | 6F |

The ZENFLEX® Peripheral Stent System is indicated for improving luminal diameter in the treatment of patients with de novo or restenotic native lesion(s) of the iliac artery, superficial femoral artery and/or proximal popliteal artery with total lesion length up to 150 mm and with a reference vessel diameter ranging from 3 mm to 8 mm.