the core of our corporate values
Seeing is Believing | First Case of Pantheris Completed in Boao, Hainan
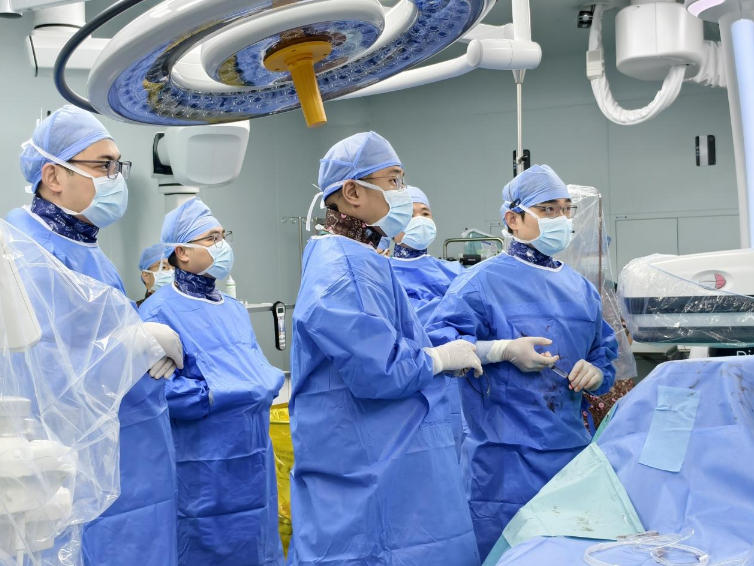
BOAO, Hainan, China – August 24, 2025 — Zylox-Tonbridge today announced that a vascular surgery team led by Prof. Jiaxuan Feng, Chief of Vascular Surgery at Ruijin Hospital affiliated with the Shanghai Jiao Tong University School of Medicine (“Ruijin Hospital”), successfully performed China’s first real-time OCT–guided atherectomy at Ruijin-Hainan Hospital affiliated with the Shanghai Jiao Tong University School of Medicine (“Hainan Boao Research Hospital”, hereinafter referred to as “Ruijin-Hainan Hospital”).
As a co-investigator under the special-access program for innovative medical devices, Prof. Youfei Qi, Chief of Vascular Surgery at Hainan Provincial People’s Hospital, participated and co-performed the procedure.
Using the Pantheris OCT-guided atherectomy system, the team performed a “visualized” plaque debulking in a patient with arterial restenosis following a previous lower-limb arterial intervention.
This first-in-China clinical use marks the introduction of the world’s first and only Lumivascular platform for peripheral intervention into China—opening a new treatment pathway for patients and offering vascular surgeons a powerful new option for precision therapy nationwide.

Seeing Is Believing: Shaping the Future of Lumivascular platform for peripheral intervention
Pantheris is the first-in-class and only “OCT-Guided” atherectomy system for peripheral intervention, integrating directional atherectomy with real-time intraluminal OCT (optical coherence tomography) imaging in a single device. In November 2024, it was granted the “Innovative Medical Device designation” from China’s National Medical Products Administration (NMPA).
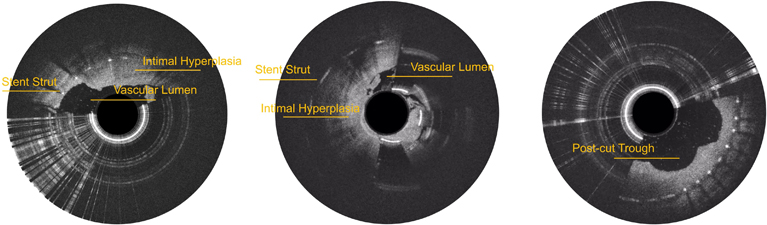
Compared with conventional interventional imaging, which provides only two-dimensional vessel imaging, real-time OCT imaging with Pantheris enables direct visualization of intraluminal structures, clear differentiation of plaque from healthy vessel wall, and safer, more precise OCT-guided atherectomy. The technology is designed to improve procedural efficiency and reduce radiation exposure for both operators and patients.
The platform offers above-the-knee and below-the-knee configurations and carries a U.S. FDA approval for treating in-stent restenosis (ISR) , addressing diverse clinical needs. Multiple international clinical studies have reported positive outcomes with OCT-guided atherectomy.
Accelerating Clinical Adoption to Benefit More Patients
In patients with lower-extremity peripheral arterial disease (PAD), ISR after prior stenting often leads to recurrent claudication and activity limitation. In these cases, conventional atherectomy can be challenging given the potential for stent-strut disruption and vessel injury.
For this high-risk clinical scenario, Prof. Feng’s team employed the Pantheris under real-time OCT guidance to distinguish plaque from stent struts and native vessel wall, then performed OCT-guided atherectomy. The intervention re-established flow without compromising stent integrity or causing vascular injury. Following the procedure, the patient recovered smoothly, with reduced pain, improved mobility, and an improved quality of life.
Pre-procedural DSA |
Post-procedural DSA |
“As an image-guided directional atherectomy system, Pantheris enables intra-procedural visualization of the vessel wall–plaque interface and supports more precise plaque excision,” said Prof. Jiaxuan Feng, Chief of Vascular Surgery at Ruijin Hospital. “It is currently the only directional atherectomy system with a U.S. FDA indication for the treatment of in-stent restenosis (ISR). Published international studies have reported favorable safety and effectiveness, and we look forward to broader clinical adoption in China.”
“Leveraging the Boao special-access framework, we can introduce advanced OCT-guided interventional technologies more rapidly, and real-time intraluminal visualization supports more precise, safer interventions for patients in Hainan and across China, with the potential to reduce recurrence and improve quality of life,” said Prof. Youfei Qi, Chief of Vascular Surgery at Hainan Provincial People’s Hospital.
“Boao’s special-access policy for innovative medical devices facilitates the domestic introduction of internationally advanced technologies, and the clinical adoption of this first-in-class and currently unique system is expected to advance precision, ‘visualized’ treatment of peripheral arterial disease in China,” said Dr. Jun Meng, Vice President of Ruijin Hainan Hospital.
Sustained Innovation to Lead Future Development
Zylox-Tonbridge stated that the successful first clinical use of Pantheris at Ruijin Hainan Hospital fills a domestic application gap and offers a new option for complex lower-limb disease. The Company is accelerating domestic manufacturing to bring this technology to more patients in China.
In parallel, Tigereye ST—which received NMPA Innovative Medical Device designation alongside Pantheris—is scheduled to enter clinical use. Tigereye ST is a OCT-guided chronic total occlusion (CTO) crossing catheter. With real-time OCT guidance, physicians can navigate within the true lumen, helping reduce subintimal entry risk and enabling efficient, safe crossing of complex CTOs to support subsequent endovascular therapy.
Looking ahead, Zylox-Tonbridge will remain committed to its mission of “Innovation for Quality Life”, advancing clinically driven innovation and delivering high-quality, accessible medtech solutions to raise the standard of vascular intervention in China.








.gif)
.gif)