the core of our corporate values
Zylox-Tonbridge’s ZYLOX Venous Stent System Receives NMPA Marketing Approval
On January 22nd, Zylox-Tonbridge Medical Technology Co., Ltd. (2190.HK, hereinafter referred to as "Zylox-Tonbridge" or the "Company") announced that its ZYLOX Peripheral Venous Stent System , a product self-developed by the Company, has received marketing approval from the National Medical Products Administration (the "NMPA"). This significant milestone in Zylox-Tonbridge's deployment of a total solution for the interventional treatment of peripheral venous diseases reinforces Zylox-Tonbride's position in the peripheral vascular intervention devices market.
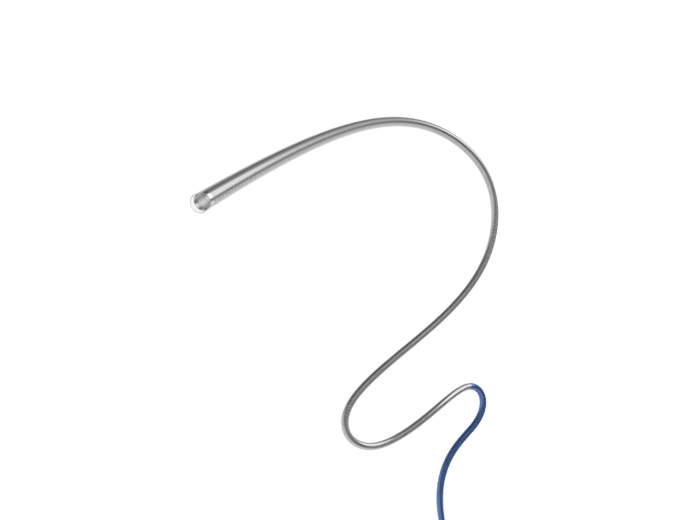
The ZYLOX Peripheral Venous Stent System is an innovative product developed by the medical-industrial joint effort of Zylox-Tonbridge in cooperation with Chinese well-known medical experts. The product has 8 invention patents and is designed for lower limb and pelvic venous return disorders caused by iliac vein compression. The ZYLOX Peripheral Venous Stent System, featuring three major designs of oblique entrance, tapered gradient, and integrated structure, is designed to reduce the risk of thrombosis while ensuring alignment with the natural diameter of the blood vessel. ZYLOX is designed to provide excellent wall adherence and gradual expansion, with a proximal closed-loop structure for strong support and a distal open-loop structure for excellent alignment.
The pre-market clinical study of the ZYLOX Peripheral Venous Stent System was led by Professor Zhao Yu of the First Affiliated Hospital of Chongqing Medical University. A total of 161 patients from 14 top clinical trial centers across the PRC were enrolled over a period of 10 months, with the target vessel patency rate reaching 100% at 12 months after the procedure. The excellent clinical trial results clearly demonstrate the safety and efficacy of this product.
May-Thurner Syndrome (MTS) is a vascular disorder where the left iliac vein gets compressed by the overlying right iliac artery, causing circulatory issues. Symptoms include leg pain, swelling, and occasionally deep vein thrombosis (DVT). Treatment involves medication, surgery, or vascular intervention, commonly using stents to alleviate compression and restore normal blood flow.
May-Thurner Syndrome (MTS) is recognized as a significant healthcare concern affecting a notable portion of the global population. Epidemiological studies, such as those conducted by the World Health Organization (WHO) and various academic institutions, suggest a substantial prevalence of MTS cases worldwide, indicating a growing need for interventions such as iliac vein stenting. With increasing awareness and diagnosis of MTS, the demand for effective treatments is expected to rise steadily[1].
With the launch of the ZYLOX Peripheral Venous Stent System, Zylox-Tonbridge aims to make a significant contribution to meeting this demand. The company is committed to providing advanced solutions for the treatment of MTS, ensuring accessibility and high standards of care for patients around the world.
As of the announcement date, the company has developed a total of 57 products and product candidates, including 36 products approved in China, eight products approved in Europe, and 1 product approved in Brazil. Zylox-Tonbridge has become one of the most competitive domestic vascular interventional medical device platforms with comprehensive product solutions. Looking forward, Zylox-Tonbridge remains committed to developing innovative products with significant clinical value to provide patients with high-quality and affordable solutions.
References:
[1] Knuttinen MG, Naidu S, Oklu R, et al. May-Thurner Syndrome: History of understanding and need for defining population prevalence. J Thromb Thrombolysis. 2014;37(3): 231-5. doi: 10.1007/s11239-013-1023-8.